The lithium-metal device replaces the typical electrode used to store lithium ions during charging with an ion-conductive substrate.
October 20, 2022

Researchers have found a way to get nearly 400 miles on a single charge of an electric vehicle (EV) by creating a new lithium-ion battery without an anode.
A research team led by Professor Soojin Park and PhD candidate Sungjin Cho in the Department of Chemistry at Ulsan Institute of Science and Technology (UNIST) of Pohang University of Science & Technology (POSTECH) in South Korea developed the battery, which in lab showed an energy density that would allow it to run for 630 kilometers, or about 391 miles, on a single charge, researchers said.
Researchers collaborated with UNIST chemical engineers Professor Dong-Hwa Seo and Dr. Dong Yeon Kim on the battery, which replaces the typical graphite anode that's used to store lithium ions during charging with an ion-conductive substrate, researchers said.
Current lithium-ion battery designs are reaching their limitations in terms of energy density, which is how much energy can be stored versus the amount of space it takes up in a battery. Current lithium-ion designs get heavy as energy density is increased, which is not a desirable feature for EV manufacturers when considering their addition to new vehicle designs.
Given this, scientists have been seeking various alternatives to traditional lithium-ion chemistries for novel EV battery designs. Anode-free batteries are one option that they've been exploring for more powerful batteries that allow EVs to travel further on a single charge without significantly affecting the weight of the vehicle, researchers said.
The Approach
Researchers at UNIST took an anode-free approach with a lithium-metal battery, which it said is “one of the finest prospects for increasing energy density beyond that of standard lithium-ion batteries,” they wrote in an abstract for a paper published on the research in the journal Advanced Functional Materials.
Batteries usually change the structure of anode materials as lithium ions flow to and from the electrode during repetitive charging and discharging. This is why the battery capacity decreases over time.
The scientific thought behind removing the anode is that if this is done—effectively charging and discharging the device only with a bare anode current collector—then the energy density would increase.
However, once executed in the lab, it was found that this method has a critical weakness--significant swelling of the anode volume and also a reduction in the battery lifecycle, researchers said. Further, they found that the swelling occurred because there was no stable storage for lithium in the anode.
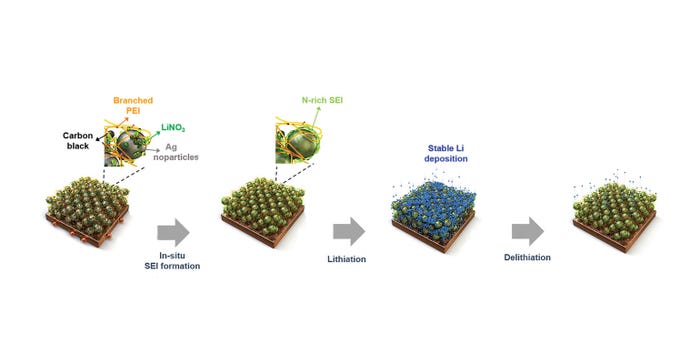
Solving the Problem
The POSTECH team managed to solve this problem in their device by creating an electrode by coating an ion conductive layer composed of polyethyleneimine polymer, silver, lithium salt, and carbon black on the surface of a copper current collector to create the substrate, they said.
This allows the battery to effectively receive and release lithium ions during charging and discharging without needing a separate anode, they said.
The substrate not only forms an anode protective layer but also helps minimize the bulk expansion of the anode, solving the critical issue that has been hindering production of anode-free batteries, researchers said.
"The ion-conducting layer causes stable solid electrolyte interphase development and allows for minimal volume expansion when utilizing stable Li hosts," they wrote in the abstract.
Tests showed that the battery has a volumetric energy density of 977 Wh/L—higher than the conventional batteries, which typically have a density of 700wh/L, researchers reported. This means that the battery can run for 630km on a single charge, they said.
Moreover, the battery also demonstrated that it can maintain a high capacity of 4.2mAh cm-2 and high current density of 2.1 mA cm-2 for a long period in the carbonate-based liquid electrolyte, researchers said. It was also proven both in theory and through experiments that the substrates can store lithium.
These features together can both help accelerate the commercialization of non-explosive batteries for EVs, researchers said.
Elizabeth Montalbano is a freelance writer, journalist and therapeutic writing mentor with more than 25 years of professional experience. Her areas of expertise include technology, business and culture.
About the Author(s)
You May Also Like





