Progress on solid-state lithium batteries is a long road filled with small steps.
February 4, 2021
It’s fun to think of scientific progress as a series of eureka moments, created by a solitary researcher toiling away in a laboratory, struck by a flash of brilliance. That’s not the way it happens of course—more often than not science is made up of thousands of tiny results, each peer-reviewed and published. Any one step can trigger hundreds or even thousands of new ideas that can be a dead-end, further the field, or open up brand new areas for research and development.
The Solid State Example
Let’s consider solid-state lithium-based batteries. They have been the subject of intense study for well over a decade. Using a lithium metal anode (negative electrode) they promise power densities of up to 2.5 times commercial lithium-ion batteries, while having the potential to be safer, allow for faster charging, and exhibit longer life cycles.
In a present-day commercial lithium-ion battery, a graphite anode (negative electrode) collects lithium ions during charging in the spaces between the layers of carbon atoms, in a process called intercalation. When the battery is discharging, the lithium ions flow through a liquid electrolyte made up of organic solvents. When the lithium ions reach the cathode (positive electrode) they intercalate into the structure of the cathode material. In high energy lithium-ion cells, the cathode is made from a combination of nickel, cobalt, and manganese (NCM), or aluminum (NCA). Lower energy cells use a lithium iron phosphate (LFP) cathode material which is both cheaper and more able to withstand abuse. The organic solvents in the electrolyte liquid are flammable and can result in a fire if the battery cells are damaged or suffer from overheating through overly fast charging or discharging.
The limiting factor for energy density in a lithium-ion battery is the intercalation of the lithium ions into and out of the graphite anode. Only a limited amount of lithium can be stored and how quickly the ions move through the three-dimensional layers limits how much power can be produced.
Lithium Metal
One way around the anode issue is to use a strip of lithium metal. When the battery is discharged a much higher number of lithium ions are available to take part in the electrochemical reactions. When charging, instead of intercalation into the graphite layers, lithium plates onto the surface of the anode. This can be a problem, however, as minor imperfections cause non-uniform plating, and the lithium can form into needle-like dendritic lithium crystals. These dendrites can grow large enough to span the distance through the electrolyte to the cathode, shorting out the battery and causing a fire.
That’s where a solid electrolyte comes into the picture. By replacing the liquid solvents with a polymer or ceramic solid, it is possible to suppress the growth of the lithium metal dendrites on the surface of the anode during charging. The solid electrolyte material must be capable of allowing lithium ions to flow between the electrodes during charging and discharging and, ideally should be capable of allowing the battery to be manufactured on existing commercial equipment.
There Are Always Challenges
Why aren’t we using solid-state batteries today? There are a variety of challenges that still must be overcome, such as cracking of the often brittle solid electrolyte and increased weight and cost of the cell materials. As always, it’s the details that count. Thousands of researchers, working in university and industry laboratories around the world are moving solid-state technology forward, one tiny step at a time.
Clean Interfaces
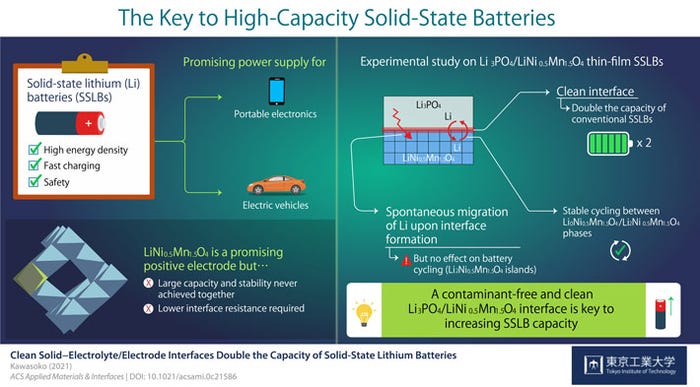
For example, a recent report from Tokyo Tech details improvements that are possible in a solid-state lithium battery (SSLB) when the interface of the surface of the cathode with the solid electrolyte is fabricated in a vacuum. In the study, the team used an oxide cathode composed of lithium, nickel, and manganese along with a solid electrolyte of lithium phosphate.
Professor Taro Hitosugi from Tokyo Tech, who led the study, explained in a news release that, "LiNi0.5Mn1.5O4 (LNMO) is a promising material for the positive electrode of SSLBs because it can generate comparatively higher voltages. In this study, we showed battery operations at 2.9 and 4.7 V, and simultaneously achieved large capacity, stable cycling, and low resistance at the electrolyte/electrode interface."
In previous work, the Tokyo Tech team had noted that producing a clean electrolyte/electrode interface was essential to achieve low interface resistance and fast charging when using LNMO cathodes in solid-state lithium batteries. They also observed that Li ions, “…spontaneously migrated from Li3PO4 (LPO) electrolyte to the LNMO layer upon fabrication, forming a Li2Ni0.5Mn1.5O4 (L2NMO) phase in LNMO with unknown distribution and impact on battery performance.”
The team examined the L2NMO, analyzing the changes in crystalline structure between the Li0Ni0.5Mn1.5O4 (L0NMO) and L2NMO phases during charging and discharging. They also studied the initial distribution of L2NMO phases at a clean LPO/LNMO interface, fabricated in a vacuum, and examined the effect of electrode thickness.
Results, One Step at a Time
The team found that “The clean interface facilitated the intercalation and deintercalation of Li during charging and discharging of the SSLBs. As a result, the capacity of SSLBs with a clean interface was twice that of conventional LNMO-based batteries. Moreover, this study marked the first time stable reversible reactions were found between the L0NMO and L2NMO phases in SSLBs.”
This was not a eureka moment. But it was one more small step in the long research path that may ultimately result in improved battery solid-state battery designs and fabrication techniques, with increased capacity, stability, and safety for cellphones, mobile electronic devices, and electric vehicles. Assistant Professor Hideyuki Kawasoko of Tohoku University, part of the research team, said, "Our findings indicate that the formation of a contamination-free, clean LPO/LNMO interface is key to increasing the capacity of SSLBs while ensuring low interface resistance for fast charging." Yet another step.
Kevin Clemens is an engineering consultant who has worked on automotive and environmental projects for more than 40 years.
About the Author(s)
You May Also Like





