A study suggests that stress caused by misaligned particles in lithium-ion electrodes limits flow—more porous electrodes might provide improvements.
April 19, 2022
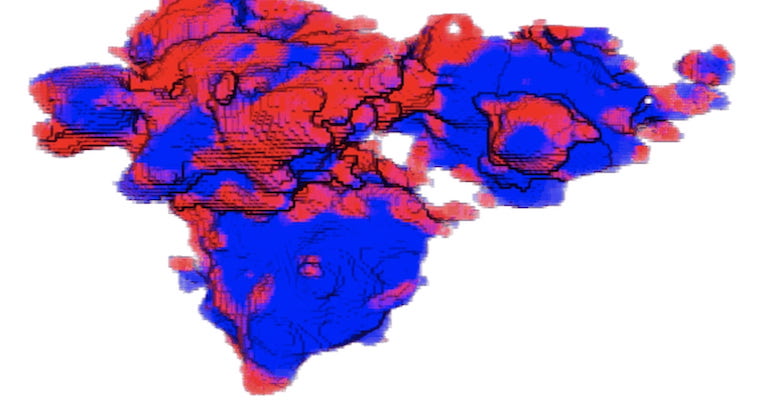
The level of charge at every scale across a lithium-ion battery electrode is highly uneven.
This is not good for the battery's health. At least that is the conclusion reached by Rice University researchers who recognized the problem and worked with the Department of Energy to view in great detail how the various particles in an electrode interact with lithium during use.
Rice scientists Ming Tang analyzed nano- and micro-scale interactions within lithium iron phosphate cathodes through modeling and imaging offered by the transmission X-ray microscopy capabilities at Brookhaven National Laboratory and Argonne National Laboratory.
Their paper, recently published in the American Chemical Society journal ACS Energy Letters supports the theories Ming Tang, an associate professor of materials science and nanoengineering at Rice University, and his colleagues formed several years ago. Their theory foresaw how lithium travels in the dynamic environment inside a typical commercial cathode.
"Batteries have a lot of particle aggregates that soak up and give up lithium, and we wanted to know what happens on their surfaces, how uniform the reaction is," said Tang in a Rice news release. "In general, we always want a more uniform reaction so we can charge the battery faster."
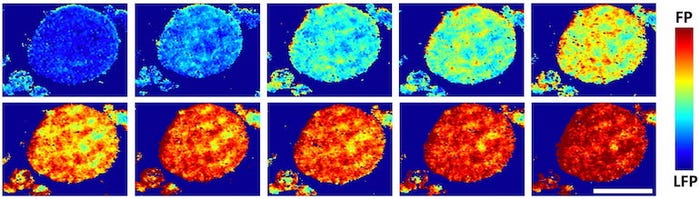
Using Brookhaven's powerful X-ray synchrotron, the researchers saw some regions inside the cathode were better at absorption than others. The ability to look at single or aggregated particles in 3D showed that rather than reacting over their entire surfaces, lithium favored particular regions over others. Being able to watch sealed cathodes charge and discharge at Brookhaven offered absolute proof.
"This is very different from conventional wisdom," Tang said. "The most interesting observation is that these reaction regions are shaped like one-dimensional filaments lying across the surface of these aggregated particles. It was kind of weird, but it matched what we saw in our models."
Tang said the lithium filaments looked something like thick nanotubes and were several hundred nanometers wide and several microns long.
According to Tang, misaligned crystallites in the particle agglomerates prevent lithium from being uniformly inserted into or extracted from the aggregate surface because that will generate too large an energy penalty. Instead, lithium is forced to flow into or out of the aggregates at "hot spots" that develop the filament shape. "This is a bad thing," Tang said, "Because the lithium can't go into the cathode uniformly, it slows down the intercalation mechanics.”
Making the lithium insertion more uniform could improve battery performance. "What our study offers is some potential ways to help make lithium insertion or extraction more uniform on these aggregates or individual particles," he said. "Introducing some porosity in the particle agglomerates might sacrifice some energy density, but at the same time would allow lithium to go in more uniformly. That could allow you to get more energy at a given charge/discharge rate.
"Another thought is if we can somehow align the orientation of these small particles so their maximum expansion is perpendicular to each other, they'll better accommodate lithium intercalation," he said.
That would be a challenge for battery manufacturers, "We don't have enough experience in synthesis to know how to make that happen," Tang said. "What we're providing is bait. Let's see if somebody bites."
Kevin Clemens is a Senior Editor with Battery Technology.
About the Author(s)
You May Also Like




