X-Ray Movies, Computer Vision Bring Battery Breakthrough
A method to improve Li-ion battery efficiency at the electrode is the result of machine learning—and 18 years of computer modeling work.

Researchers have harnessed computer vision, a form of machine learning, to gain new insights into the workings of rechargeable lithium-ion batteries. By meticulously analyzing X-ray movies of the battery electrodes at the nanoscale level, scientists have uncovered physical and chemical details that were previously hidden. This breakthrough has the potential to enhance the efficiency of lithium-ion batteries, and the findings could have broader applications in understanding complex systems like cell division in developing embryos.
The study, led by researchers from the Department of Energy's SLAC National Accelerator Laboratory, Stanford University, the Massachusetts Institute of Technology (MIT), and the Toyota Research Institute, focused on lithium iron phosphate (LFP) particles. LFP particles, coated with a thin layer of carbon for improved electrical conductivity, are found in the positive electrodes of many lithium-ion batteries.
Transparent battery cells
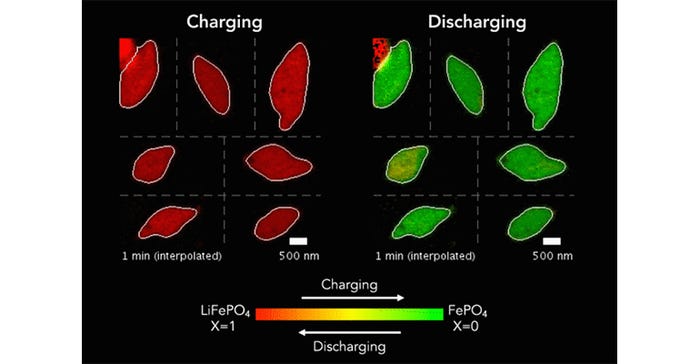
To observe the battery's inner workings, the research team created transparent cell batteries with two electrodes surrounded by an electrolyte solution containing free-moving lithium ions. As the battery discharges, lithium ions enter the positive LFP electrode and become lodged within its nanoparticles, akin to cars in a crowded parking garage—a process called intercalation. When the battery charges, these ions flow out and move to the negative electrode.
LFP is a crucial battery material known for its low cost, safety record, and use of abundant elements, making it particularly relevant in the electric vehicle market.
The collaboration between researchers began eight years ago when MIT Professor Martin Bazant and Stanford's William Chueh combined their expertise in mathematical modeling and advanced X-ray microscopy to study battery particles. They later incorporated machine learning tools to accelerate battery testing and identify optimal charging methods. This latest study leveraged computer vision to delve into the details of 62 nanoscale X-ray movies from 2016, each containing around 490 pixels, to gain a more comprehensive understanding of lithium insertion reactions within LFP particles.
Using this microscopy technique, researchers can capture images pixel by pixel, revealing the concentration of lithium ions at each point within the particle. They can scan the particles multiple times during charging and discharging, enabling the creation of movies illustrating the flow of lithium ions in and out of the particles.
In 2017, Bazant and his colleagues at SLAC received funding from the Toyota Research Institute to conduct further studies using this approach, alongside other battery-related research projects.
From movies to models
By analyzing X-ray images of the lithium iron phosphate particles as they underwent charging and discharging cycles, the researchers discovered that the movement of lithium ions within the material closely matched the computer simulations previously created by Bazant. Using 180,000 pixels as measurements, they trained a computational model to generate equations accurately describing the nonequilibrium thermodynamics and reaction kinetics of the battery material.
Bazant explains that “every little pixel in there is jumping from full to empty, full to empty, and we're mapping that whole process, using our equations to understand how that's happening.”
Furthermore, the study revealed that the observed patterns of lithium-ion flow could unveil spatial variations in the rate at which lithium ions are absorbed at different locations on the particle's surface. This discovery led to the identification of correlations between these variations and the thickness of the carbon coating on the surface of the lithium iron phosphate particles. This carbon coating is applied to enhance the material's electrical conductivity, a critical factor in battery performance.
What it means for battery design
The study's results suggest that optimizing the thickness of the carbon layer on the electrode's surface could potentially enhance the efficiency of batteries, marking a significant advancement in battery design. The findings also lend quantitative support to a hypothesis previously formulated by Bazant: that the performance of lithium iron phosphate electrodes is predominantly limited by the rate of coupled ion-electron transfer at the interface between the solid particle and the carbon coating, rather than the rate of lithium-ion diffusion within the solid.
Beyond batteries, Bazant believes that this analytical approach could be invaluable for studying pattern formation in other chemical and biological systems. Overall, this research demonstrates the potential of machine learning and advanced imaging techniques to uncover profound insights into materials and systems, offering opportunities for the advancement of battery technology—and beyond.
The new study is available in the journal Nature and was first summarized in news releases from MIT and Stanford University.
About the Author(s)
You May Also Like





