Advanced Binders for Ultra-Thin Sulfide Solid State Batteries
Discover groundbreaking research on advanced binders enabling ultra-thin sulfide solid electrolyte membranes for high-performance all-solid-state batteries.
February 6, 2024
“The development of thin sulfide solid electrolyte layers is imperative” stated paper author Xiayin Yao, a professor at the Laboratory of All-solid-state rechargeable battery, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences (CAS). “Although sulfide solid electrolyte is easily densified through the cold pressing method, the freestanding membrane generally shows a thickness greater than 500 μm. The thick and heavy layer of sulfide electrolyte results in less than expected cell-level energy density.”
Yao explained the difficulty in developing ultra-thin sulfide electrolyte membranes, suffering fracture during the cycle due to their brittleness nature, leading to short circuits of the battery.
Innovations in binder technology
“Inspired by the binder-assisted electrode fabrication in conventional lithium-ion batteries,” Yao stated. “Combining polymeric binders with sulfide solid electrolytes is a promising strategy to prepare thin sulfide solid electrolyte layers with high mechanical strength."
Nevertheless, a slurry-based system has not yet been established due to the sensitivity character of sulfide solid electrolyte particles. Generally, desirable polymeric binders should possess sufficient adhesion capability to adapt to the volume changes during cell cycling while maintaining the performance of the sulfide solid electrolyte. Meanwhile, considering the chemical instability of sulfide solid electrolytes, the solvents are restricted to weakly polar organic solvents, such as toluene, xylene, heptane, ethyl acetate, etc. It is challenging to balance the stability and suitable adhesion between sulfide solid electrolyte particles and polymeric binders.
“The design of polymer binders with suitable solvents is rarely reported for the preparation of sulfide solid electrolyte membranes through the wet method,” Yao explained. “The polarity of the solvent is the primary factor to consider in its compatibility with sulfide solid electrolytes, which is related to the functional groups contained and molecular structure. However, for different sulfide solid electrolytes, the polarity cannot reflect the same compatibility of electrolyte with solvents.”
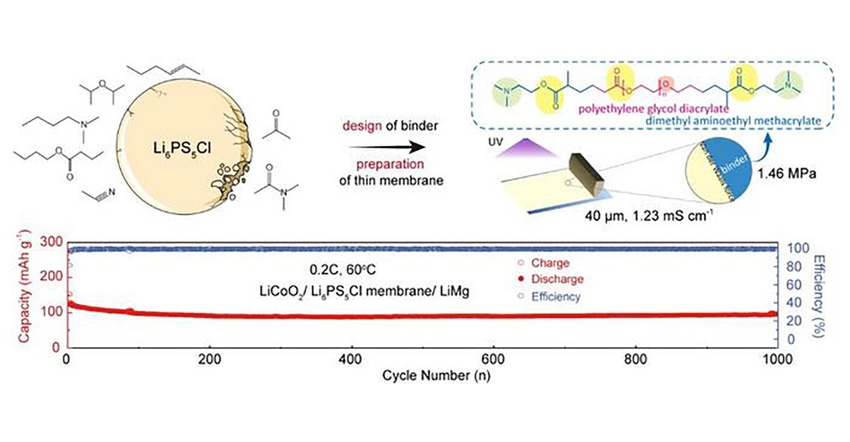
“It is urgent to develop advanced binders with suitable solvents to realize ultrathin, robust, and highly ionic conductivity sulfide solid electrolyte membranes.” Yao continued. “In this paper, the interaction among solvents containing different functional groups with the Li6PS5Cl solid electrolyte was explored, and a new polymeric binder with superior mechanical properties and excellent stability to Li6PS5Cl was designed.”
“Different from previous conventional binders, the precursors of polymer binder are easily dispersed in anhydrous acetonitrile. Through in-situ photo-polymerization, a free-standing Li6PS5Cl thin membrane with room temperature ionic conductivity of 1.23 mS cm−1 can be prepared.” Yao stated.
“The assembled LiCoO2| Li6PS5Cl membrane| LiMg all-solid-state battery can stably cycle 1000 cycles at 0.2C under 60oC,” Yao concluded. The pouch-type cells still exhibit outstanding electrochemical performance when increasing the mass loading of active materials up to 15.2 mg cm−2, which provides a new slurry-based system for the preparation of Li6PS5Cl electrolyte thin membrane in all-solid-state batteries.
About the Author(s)
You May Also Like





