What Is Thermal Runaway? Here’s a Battery of Answers
If you do not use, charge, or store lithium-ion batteries in the right way you risk thermal runaway. Here’s what can be done to prevent batteries from catching fire.
A thermal runaway is an uncontrollable chain reaction in a lithium-ion battery cell that can lead to a fire hazard. In ideal conditions, the lithium-ion cells of a battery can dissipate the heat generated by the movement of electrons and ions to produce electricity. But in thermal runaway, battery cells are unstable, and temperatures rise at very fast rate, reaching extremely high values of approx. 752°F and causing materials inside the cell to start breaking down and decompose. The result can be fire, the release of toxic/flammable gases, and possible explosions.
Stages of thermal runaway:
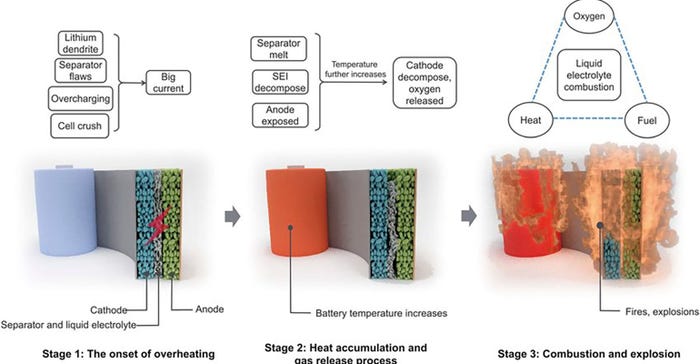
There are three stages for the thermal runaway process.
Stage 1: It stars with the onset of overheating of the battery system. The batteries change from a normal to an abnormal state due to an internal or external factor. The internal temperature starts to increase. When the internal temperature starts to increase, stage 1 ends and stage 2 begins.
Stage 2: During this stage, the separator melts, the solid electrolyte interphase (SEI) layer decomposes, and the anode is exposed. The internal temperature quickly rises, and the battery undergoes exothermal reactions—chemical reactions that produce heat—that can produce heat accumulation. With the decomposition of SEI, the temperature builds up, and the lithium metal or intercalated lithium in the anode will react with the organic solvents in the electrolyte, releasing flammable hydrocarbon gases (ethane, methane, and others).
During stage 2, as the temperature increases oxygen begins to accumulate inside batteries. The thermal runaway process proceeds from stage 2 to stage 3 as soon as enough oxygen and heat have accumulated for battery combustion.
Stage 3: The released oxygen and heat in stage 2 provide the required conditions for the combustion of flammable organic electrolytes, thereby leading to fires and even explosions.
Causes of thermal runaway
External factors and internal failures in the battery can cause thermal runaway:
Overcharging the battery: If a battery is charge over its safe maximum voltage, it can damage the battery.
Storage temperature: The ideal storage temperature for most batteries, including lithium-ion, is 40–70°F, depending on the manufacture. Exposing a battery to high temperatures can degrade battery’s performance triggering thermal runaway.
Internal short-circuit: A lithium-ion cell contains a cathode and an anode that are physically separated by a membrane called the separator. In cells of poor quality, defects can affect the separator’s integrity and can cause an internal short circuit condition that can result in thermal runaway.
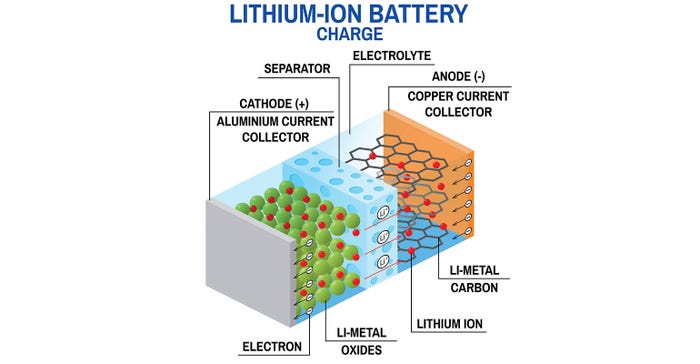
Physical damage (e.g., puncture): A puncture can also cause short circuit between the anode and cathode, leading to a spike in current at that point in the cell.
Excessively high discharge rate: Discharging the cell or battery below the cell manufacturer-recommended lower voltage threshold multiple times, then charging the cell can lead to thermal runaway.
Rapid charging: This might lead to excessive currents.
Preventing the risk of thermal runaway
Preventing thermal runaway calls for more than just storing batteries at safe temperatures and avoiding overcharging them. Research for new materials for anodes/cathodes and new designs are constantly evolving. Here are some of the technologies and techniques that are evolving and currently helping to prevent thermal runaway:
Battery Management Systems: A battery management system (BMS) is an electronic system that can manage, monitor, and control rechargeable battery packs. There are many designs and types, depending on the application; recently Battery Technology wrote an article about wireless BMS.
Avoiding cell-to-cell propagation: Preventing cell-to-cell propagation might be done by considering using enough space between cells in a pack. Also, by adopting the right heatsinks or proper venting features.
Separator shutdown: A particular separator that could potentially enhance the safety of battery by having a shutdown temperature and breakdown temperature as far apart as possible to avoid or delay the thermal runaway process.
Increasing battery performance and safety is a common concern for every business involved in battery manufacturing, especially in the electric vehicle (EV) market that has been growing steadily. We will see more innovations coming from researchers and scientists working on battery safety.
About the Author(s)
You May Also Like





