Graphite Anodes For Lithium-Ion Batteries
Graphite is the most common material used for the anode of lithium-ion batteries. Here’s why.
February 4, 2021
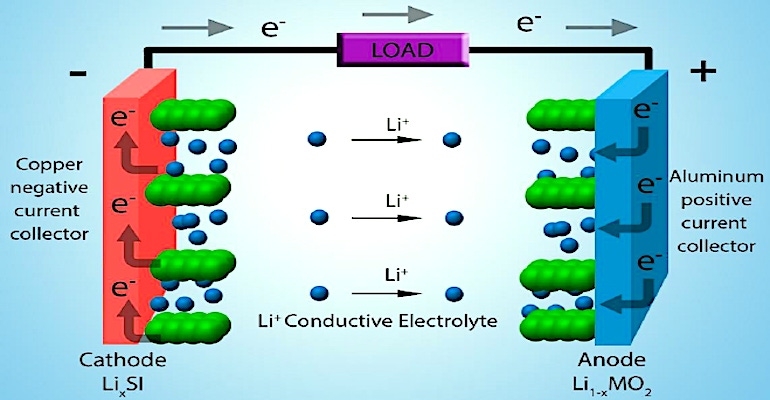
Lithium-ion batteries are made up of a variety of components. There is a positive electrode (cathode) that is usually made up of a metal oxide. There is a negative electrode (anode) that is typically a form of carbon graphite material. Between the electrodes is a liquid organic solvent electrolyte that allows the transfer of ions, and an ion-permeable plastic separator. Charge collectors (usually thin sheets of aluminum) back the cathode and anode to aid in the flow of electrons. The entire cell is made up of a long strip that is rolled up into a cylinder (jelly roll) or placed in a flexible plastic pouch or rigid plastic box-like structure.
Anodes
Let’s consider the anode. The graphite material of the anode is placed in sheets or layers and reversibly allows the placement of lithium ions into (intercalation) or out of (deintercalation) during charging and discharging, respectively. Anode materials must allow fast diffusion of lithium ions into the structure, high ionic and electron conductivity, minimal structural changes (expansion and contraction) during charging and discharging, a high capacity for lithium ions, and an ability to form and maintain a stable Solid Electrolyte Interface (SEI) during the charge and discharge cycling.
Although we call them lithium-ion batteries, lithium makes up only about 2% of the total volume of the battery cell. There is as much as 10-20 times as much graphite in a lithium-ion battery. The anode is made up of powdered graphite that is spread, along with a binder, on a thin aluminum charge collector. The anode is manufactured separately from the rest of the lithium-ion cell.
From China
The anode material begins as flake graphite, a naturally produced form of carbon. About 20% of the global mining of flake graphite feedstock undergoes intense physical milling and chemical purification to produce uncoated spherical graphite. Most of the mining of flake graphite takes place in China, scattered across the country, with several small scale mines feeding into larger processing plants.
Battery anodes require silicon oxide coated spherical graphite at over 99.9% purity and, at present, 100% of natural spherical graphite is produced in China. Synthetic or artificial graphite can also be used in anodes and when that is added into the mix, China and Japan together sell more than 95% of the total global anode materials.
In 2019, the world’s top five anode material producers were Shenzhen BTR, Jiangxi Zichen, Ningbo Shanshan, Guangdong Kaijin, and Hitachi Chemical, with a combined 69.65% share in the global market. The top 5 players in the synthetic graphite market include Shanshan Technology, Shenzhen Sinuo Industrial Development Co. Ltd, BTR New Energy Materials Inc., Jiangxi Zichen Technology Co. Ltd, and Hitachi Chemical Co. Ltd., and account for 75% of the market.
Silicon Alternative
Some alternatives are under consideration for the graphite used in lithium-ion battery anodes. Silicon as used in computer chips and electronics devices is getting a lot of attention. Potentially, silicon can hold 10 times the electrical charge per gram when compared to graphite, resulting in a battery with a much higher capacity and charge density. But the problem is when lithium ions enter the silicon structure, it expands greatly, and the volumetric changes can create cracking and disconnection of the silicon from its electron charge collector after just a few charge and discharge cycles. Silicon also can form a highly resistive SEI, reducing its ability to transfer ions. A variety of efforts are underway using stress relief through silicon nanostructures or the addition of carbon nanotubes, but nothing is ready for widespread commercial application at the moment.
Kevin Clemens is an engineering consultant who has worked on automotive and environmental projects for more than 40 years.
About the Author(s)
You May Also Like